Meprylcaine
In today's world, Meprylcaine has acquired a unique relevance that significantly impacts various aspects of daily life. Since its appearance, Meprylcaine has been the subject of discussion, analysis and controversy, generating a wide spectrum of opinions and visions around its meaning and influence. In this article, we will explore the different facets of Meprylcaine and its impact on society, culture and economy, providing a detailed analysis on its importance and role in today's world.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H21NO2 |
| Molar mass | 235.327 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Meprylcaine (also known as Epirocaine and Oracaine) is a local anesthetic with stimulant properties that is structurally related to dimethocaine.[1]
Meprylcaine has a relatively potent inhibitory action on the monoamine transporter and inhibits the reuptake of dopamine, norepinephrine and serotonin.[2][3]
Synthesis
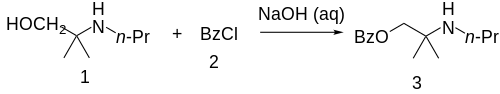
The 2-methyl-2-(propylamino)propan-1-ol (1) is treated with base and then with Benzoyl chloride (2), completing the synthesis of Meprycaine (3).
References
- ^ Sato T, Kitayama S, Mitsuhata C, Ikeda T, Morita K, Dohi T (February 2000). "Selective inhibition of monoamine neurotransmitter transporters by synthetic local anesthetics". Naunyn-Schmiedeberg's Archives of Pharmacology. 361 (2): 214–20. doi:10.1007/s002109900184. PMID 10685879. S2CID 1627097.
- ^ Arai S, Morita K, Kitayama S, Kumagai K, Kumagai M, Kihira K, Dohi T (February 2003). "Chronic inhibition of the norepinephrine transporter in the brain participates in seizure sensitization to cocaine and local anesthetics". Brain Research. 964 (1): 83–90. doi:10.1016/S0006-8993(02)04068-4. PMID 12573515. S2CID 16539422.
- ^ Morita K, Hamamoto M, Arai S, Kitayama S, Irifune M, Kawahara M, et al. (September 2005). "Inhibition of serotonin transporters by cocaine and meprylcaine through 5-TH2C receptor stimulation facilitates their seizure activities" (PDF). Brain Research. 1057 (1–2): 153–60. doi:10.1016/j.brainres.2005.07.049. PMID 16125150. S2CID 30437231. Archived from the original (PDF) on 2017-08-17. Retrieved 2019-12-11.
- ^ Reasenberg, Julian R.; Goldberg, Samuel D. (1945). "Esters of β-Alkylaminoethanols". Journal of the American Chemical Society 67 (6): 933–939. doi:10.1021/ja01222a017.
- ^ Julian R Reasenberg, U.S. patent 2,767,207 (1956 to Mizzy Inc).
- ^ Julian R Reasenberg, Samuel D Goldberg, U.S. patent 2,421,129 (1947 to Oradent Chemical Co Inc).