Tuaminoheptane
In this article, we will explore the fascinating world of Tuaminoheptane. From its origins to its applications today, Tuaminoheptane has played an important role in various areas of daily life. Through a detailed analysis, we will delve into the different aspects that make Tuaminoheptane a relevant topic worthy of investigation. From its benefits to its challenges, we will address the various perspectives surrounding Tuaminoheptane, offering a comprehensive view that will allow the reader to better understand its importance in the contemporary world. Join us on this exciting tour of Tuaminoheptane and discover everything this theme has to offer.
 | |
| Clinical data | |
|---|---|
| Trade names | Heptin, Heptadrine, Tuamine |
| Other names | Tuamine; 2-Aminoheptane; 2-Heptanamine; 1-Methylhexylamine |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.233 |
| Chemical and physical data | |
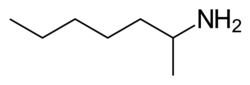
| Formula | C7H17N |
| Molar mass | 115.220 g·mol−1 |
| 3D model (JSmol) | |
| Density | 0.766 g/mL g/cm3 |
| |
| |
Tuaminoheptane (INN, BAN; brand names Heptin, Heptadrine, Tuamine; also known as tuamine and 2-aminoheptane) is a sympathomimetic agent and vasoconstrictor which was formerly used as a nasal decongestant.[2][3][4] It is still used in France as a nasal decongestant but its use is not recommended by the health authorities due to the lack of evidence of its effectiveness. It has also been used as a stimulant.[5][6]
Tuaminoheptane has been found to act as a reuptake inhibitor and releasing agent of norepinephrine, which may underlie its decongestant and stimulant effects.[7][8][6] It is an alkylamine.[6] The chemical structure of the drug differs from that of other norepinephrine releasing agents, such as the phenethylamines, which, in contrast to tuaminoheptane, have an aromatic ring in their structure.[8] Tuaminoheptane is also a skin irritant and can cause contact dermatitis via inhibition of volume-regulated anion channels, which limits its usefulness as a decongestant.[9]
Tuaminoheptane is on the 2011 list of prohibited substances published by the World Anti-Doping Agency.[5]
See also
- 1,3-Dimethylbutylamine
- 1,4-Dimethylamylamine
- Heptaminol
- Iproheptine
- Isometheptene
- Methylhexanamine
- Octodrine
- Oenethyl
References
- ^ "tuamine - Compound Summary". PubChem. USA: National Center for Biotechnology Information. 25 March 2005. Identification and Related Records. Retrieved 31 May 2012.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 623–. ISBN 978-1-4757-2085-3.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 282–. ISBN 978-94-011-4439-1.
- ^ Nickerson M, Dresel PE (September 1958). "Adrenergic drugs and their antagonists". Postgraduate Medicine. 24 (3): 246–256. doi:10.1080/00325481.1958.11692208. PMID 13591086.
- ^ a b Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". British Journal of Pharmacology. 154 (3): 606–622. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- ^ a b c Thevis M, Sigmund G, Geyer H, Schänzer W (March 2010). "Stimulants and doping in sport". Endocrinology and Metabolism Clinics of North America. 39 (1): 89–105, ix. doi:10.1016/j.ecl.2009.10.011. PMID 20122452.
- ^ Delicado EG, Fideu MD, Miras-Portugal MT, Pourrias B, Aunis D (August 1990). "Effect of tuamine, heptaminol and two analogues on uptake and release of catecholamines in cultured chromaffin cells". Biochemical Pharmacology. 40 (4): 821–825. doi:10.1016/0006-2952(90)90322-c. PMID 2386550.
- ^ a b Schlessinger A, Geier E, Fan H, Irwin JJ, Shoichet BK, Giacomini KM, Sali A (September 2011). "Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET". Proceedings of the National Academy of Sciences of the United States of America. 108 (38): 15810–15815. Bibcode:2011PNAS..10815810S. doi:10.1073/pnas.1106030108. PMC 3179104. PMID 21885739.
- ^ Raoux M, Colomban C, Delmas P, Crest M (June 2007). "The amine-containing cutaneous irritant heptylamine inhibits the volume-regulated anion channel and mobilizes intracellular calcium in normal human epidermal keratinocytes". Molecular Pharmacology. 71 (6): 1685–1694. doi:10.1124/mol.106.033324. PMID 17384225. S2CID 29565968.