Nalmexone
In this article we are going to address the topic of Nalmexone, which has gained relevance in recent years due to its impact in different areas. Nalmexone has been the subject of debate and analysis by experts in the area, who have highlighted the importance of understanding and reflecting on its implications. Throughout this article, we will examine different perspectives and research related to Nalmexone, with the goal of providing a comprehensive and up-to-date view on this topic. Likewise, we will explore its influence on society, the economy, politics and other relevant aspects, in order to understand its scope and impact in the current context.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H25NO4 |
| Molar mass | 355.434 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
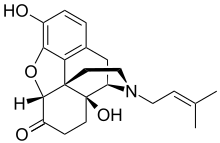
Nalmexone (INN; also known as nalmexone hydrochloride (USAN) or by the development codes EN-1620A and UM-592) is a semisynthetic, opioid partial agonist or mixed agonist-antagonist with both analgesic and narcotic antagonist properties that was never marketed.[1][2][3][4] In clinical studies it was found to have comparable analgesic efficacy to morphine, though with several-fold reduced potency.[5] In addition, nalmexone's side effects, the most common of which were sleepiness and sweating, were reported to be similar to those of morphine, albeit with a noticeably higher degree of incidence.[5]
Synthesis
Nalmexone can be synthesized from oxymorphone:[6]
See also
References
- ^ Macdonald F (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1395. ISBN 978-0-412-46630-4. Retrieved 11 May 2012.
- ^ Casy AF, Parfitt RT (1986). Opioid Analgesics: Chemistry and Receptors. Springer. p. 55. ISBN 978-0-306-42130-3. Retrieved 11 May 2012.
- ^ Loew GH, Berkowitz DS (1978). "Quantum chemical studies of N-substituent variation in the oxymorphone series of opiate narcotics". Journal of Medicinal Chemistry. 21 (1): 101–106. doi:10.1021/jm00199a018. PMID 73588.
- ^ Forrest WH, Shroff PF, Mahler DL (1972). "Analgesic and other effects of nalmexone in man". Clinical Pharmacology and Therapeutics. 13 (4): 520–525. doi:10.1002/cpt1972134520. PMID 4557582. S2CID 30780581.
- ^ a b Committee on Problems of Drug Dependence (1969). Bulletin, problems of drug dependence. National Academies. p. 5873. NAP:10503. Retrieved 11 May 2012.
- ^ Lowenstein, M. J.; Fishman, J.; 1967, U.S. patent 3,320,262
