Tantalum(IV) iodide
In this article, we will explore the fascinating world of Tantalum(IV) iodide and everything that this concept encompasses. From its origins to its relevance today, we will delve into key aspects that will allow us to thoroughly understand Tantalum(IV) iodide and its impact in different areas. Through a thorough analysis and critical perspective, we will discover the importance of Tantalum(IV) iodide in our current society and how it has evolved over time. From its implications in popular culture to its influence on the global economy, Tantalum(IV) iodide is a topic that deserves to be explored in depth to understand its scope and relevance in today's world.
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| TaI4 | |
| Molar mass | 688.57 |
| Appearance | black solid[1] |
| Melting point | 398 °C (671 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tantalum(IV) iodide is an inorganic compound with the chemical formula TaI4. It dissolves in water to give a green solution, but the color fades when left in the air and produces a white precipitate.[2]
Preparation
Tantalum(IV) iodide can be prepared by the reduction reaction of tantalum(V) iodide and tantalum.[2] If pyridine is used as the reducing agent, there is an adduct TaI4(py)2.[3]
Tantalum(IV) iodide can also be obtained by reacting tantalum(V) iodide with aluminum, magnesium or calcium at 380 °C. Ta6I14 is also formed. This makes it difficult to produce a very pure crystallized tantalum(IV) iodide.[4]
- 3 TaI5 + Al → 3 TaI4 + AlI3
Properties
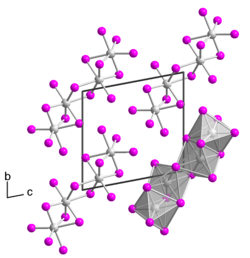
Tantalum(IV) iodide is a black solid. It has a crystal structure isotypic to that of niobium(IV) iodide.[4] Single-crystalline tantalum(IV) iodide was first obtained in 2008 by Rafal Wiglusz and Gerd Meyer as a chance product of a reaction in a tantalum ampoule that was supposed to lead to the product Rb(Pr6C2)I12.[5] The single crystal has a triclinic crystal structure with space group P1 (space group no. 2) with two formula units per unit cell (a = 707.36 pm, b = 1064.64 pm, c = 1074.99 pm, α = 100.440°, β = 89.824° and γ = 104.392°). The crystal structure differs from that of other transition metal tetraiodides, which usually have a MI4/2I2/1 chain structure, as it consists of TaI6 octahedra bridged over a common surface to form a dimer. Two such dimers bridge over a common edge to form a tetramer.[6]
References
- ^ Georg Brauer: Handbuch der präparativen anorganischen Chemie. 3., umgearb. Auflage. Band III. Enke, Stuttgart 1981, ISBN 3-432-87823-0, pp. 1455.
- ^ a b Robert F. Rolsten (Jun 1958). "Preparation and X-ray Study of Some Tantalum Halides". Journal of the American Chemical Society. 80 (12): 2952–2953. doi:10.1021/ja01545a011. ISSN 0002-7863. Retrieved 2021-03-24.
- ^ R. E. McCarley, J. C. Boatman (Jun 1963). "The Preparation of Tantalum(IV) Bromide, Tantalum(IV) Iodide, and Pyridine Adducts of the Tantalum(IV) Halides". Inorganic Chemistry. 2 (3): 547–551. doi:10.1021/ic50007a030. ISSN 0020-1669. Retrieved 2021-03-24.
- ^ a b Handbuch der präparativen anorganischen Chemie. 3 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1981. ISBN 978-3-432-87823-2.
- ^ Meyer, Gerd; Wiglusz, Rafal; Pantenburg, Ingo; Mudring, Anja-Verena (May 2008). "Tantalum(IV) Iodide, TaI4: A Molecular Solid Consisting of Dimers of Dimers, Ta4I16". Zeitschrift für anorganische und allgemeine Chemie (in German). 634 (5): 825–828. doi:10.1002/zaac.200700529.
- ^ Habermehl, Katja (2010). Neue Untersuchungen an Halogeniden des Niobs und Tantals (text.thesis.doctoral thesis). Universität zu Köln.