Lanthanum(III) iodide
In this article, we will thoroughly explore Lanthanum(III) iodide and its impact on different aspects of everyday life. From its influence on society to its relevance in history, Lanthanum(III) iodide has played a crucial role that deserves to be analyzed in detail. Through a comprehensive analysis, we will examine the importance of Lanthanum(III) iodide in today's world and how it has evolved over time. Likewise, we will explore the different perspectives and opinions related to Lanthanum(III) iodide, with the aim of providing a broad and complete vision on this topic. Ultimately, this article aims to offer a deep and detailed look at Lanthanum(III) iodide and its relevance in contemporary society.
 | |
| Names | |
|---|---|
| Other names
Lanthanum triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.045 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LaI 3 | |
| Molar mass | 519.62 |
| Density | 5.63 g/mL at 25 °C |
| Melting point | 772 °C (1,422 °F; 1,045 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.[1]
Synthesis
Lanthanum(III) iodide can be synthesised by the reaction of lanthanum metal with mercury(II) iodide:[2][3]
- 2 La + 3 HgI2 → 2 LaI3 + 3 Hg
It can also be prepared from the elements, that is by the reaction of metallic lanthanum with iodine:[2]
- 2 La + 3 I2 → 2 LaI3
While lanthanum(III) iodide solutions can be generated by dissolving lanthanum oxide in hydroiodic acid, the product will hydrolyse and form polymeric hydroxy species:[4]
- La2O3 + 6 HI → 2 LaI3 + 3 H2O → further reactions
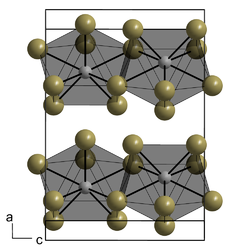
Structure
Lanthanum(III) iodide adopts the same crystal structure as plutonium(III) bromide, with 8-coordinate metal centres arranged in layers.[4][5] This orthorhombic structure is typical of the triiodides of the lighter lanthanides (La–Nd), whereas heavier lanthanides tend to adopt the hexagonal bismuth(III) iodide structure.[3]
Reactivity and applications
Lanthanum(III) iodide is very soluble in water and is deliquescent.[4] Anhydrous lanthanum(III) iodide reacts with tetrahydrofuran to form a photoluminescent complex, LaI3(THF)4, with an average La–I bond length of 3.16 Å.[6][7] This complex is a starting material for amide and cyclopentadienyl complexes of lanthanum.[6][8]
Related compounds
Lanthanum also forms a diiodide, LaI2. It is an electride and is best formulated {LaIII,2I−,e−}, with the electron delocalised in a conduction band.[4] Several other lanthanides form similar compounds, including CeI2, PrI2 and GdI2.[9] Lanthanum diiodide adopts the same tetragonal crystal structure as PrI2.[10]
Lanthanum(III) iodide reacts with lanthanum metal under an argon atmosphere in a tantalum capsule at 1225 K to form the mixed-valence compound La2I5.[11]
Reduction of LaI2 or LaI3 with metallic sodium in an argon atmosphere at 550 °C gives lanthanum monoiodide, LaI, which has a hexagonal crystal structure.[12]
References
- ^ Taylor, Moddie D. (1962). "Preparation of Anhydrous Lanthanon Halides". Chem. Rev. 62 (6): 503–511. doi:10.1021/cr60220a001.
- ^ a b Corbett, John D. & Simon, Arndt (1984). "Chapter 6: Lanthanum Triiodide (and Other Rare Earth Metal Triiodides)". In Holt Jr., Smith L. (ed.). Inorg. Synth. Vol. 22. pp. 11–16. doi:10.1002/9780470132531.ch6.
- ^ a b Asprey, L. B.; Keenan, T. K.; Kruse, F. H. (1964). "Preparation and Crystal Data for Lanthanide and Actinide Triiodides". Inorg. Chem. 3 (8): 1137–1141. doi:10.1021/ic50018a015.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 949–950. ISBN 978-0-08-037941-8.
- ^ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 420. ISBN 978-0-19-965763-6.
- ^ a b Ortu, Fabrizio (2022). "Rare Earth Starting Materials and Methodologies for Synthetic Chemistry". Chem. Rev. 122 (6): 6040–6116. doi:10.1021/acs.chemrev.1c00842. PMC 9007467. PMID 35099940.
- ^ Li, Yangjuan; Chen, Xiuting; Gong, Yu (2021). "Photoluminescence of LaI3 switched on and off by association and dissociation of non-luminescent tetrahydrofuran". Dalton Trans. 50 (11): 3797–3800. doi:10.1039/D1DT00162K. PMID 33720234. S2CID 232232544.
- ^ Windorff, Cory J.; Dumas, Megan T.; Ziller, Joseph W.; Gaunt, Andrew J.; Kozimor, Stosh A.; Evans, William J. (2017). "Small-Scale Metal-Based Syntheses of Lanthanide Iodide, Amide, and Cyclopentadienyl Complexes as Analogues for Transuranic Reactions". Inorg. Chem. 56 (19): 11981–11989. doi:10.1021/acs.inorgchem.7b01968. PMID 28915015.
- ^ Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. p. 1250. ISBN 978-0-19-965763-6.
- ^ Burrow, J. H.; Maule, C. H.; Strange, P.; Tothill, J. N.; Wilson, J. A. (1987). "The electronic conditions in the 5d1 layer-metal LaI2 making comparison with the iso-electronic tantalum dichalcogenides, with the other RE di-iodides, and with the RE monochalcogenides". J. Phys. C: Solid State Phys. 20 (26): 4115–4133. Bibcode:1987JPhC...20.4115B. doi:10.1088/0022-3719/20/26/014.
- ^ Mattausch, Hj.; Oeckler, O.; Simon, A. (2003). "Crystal structure of dilanthanum pentaiodide, La2I5". Z. Kristallogr. NCS. 218 (3): 281. doi:10.1524/ncrs.2003.218.3.281. S2CID 94059677.
- ^ Ryazanov, Mikhail; Kienle, Lorenz; Simon, Arndt; Mattausch, Hansjürgen (2006). "New Synthesis Route to and Physical Properties of Lanthanum Monoiodide". Inorg. Chem. 45 (5): 2068–2074. doi:10.1021/ic051834r. PMID 16499368.