Lonaprisan
In today's world, Lonaprisan has become a topic of great importance and interest to a wide variety of people. Whether due to its relevance in the cultural, social, scientific or technological field, Lonaprisan has become a key reference point in contemporary society. Over the years, Lonaprisan has sparked the curiosity of researchers, academics, professionals and hobbyists, generating a vast body of knowledge and debate around this topic. In this article, we will explore the multiple facets of Lonaprisan, analyzing its impact in different areas and offering a global vision of its importance and relevance today.
 | |
| Clinical data | |
|---|---|
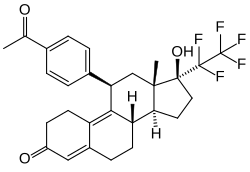
| Other names | ZK-230211; BAY 86-5044; ZK-PRA; 11β-(4-acetylphenyl)-17β-hydroxy-17α-(1,1,2,2,2-pentafluoroethyl)estra-4,9-dien-3-one |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.190.674 |
| Chemical and physical data | |
| Formula | C28H29F5O3 |
| Molar mass | 508.529 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lonaprisan (INN, USAN) (developmental code names ZK-230211, BAY 86-5044, ZK-PRA) is a synthetic, steroidal antiprogestogen which was under development by Bayer HealthCare Pharmaceuticals for the treatment of endometriosis, dysmenorrhea, and breast cancer but was discontinued.[1][2][3] It is a potent and highly selective silent antagonist of the progesterone receptor (PR).[2][3][4] The drug reached phase II clinical trials prior to its discontinuation.[1]
See also
References
- ^ a b "Lonaprisan - AdisInsight". adisinsight.springer.com.
- ^ a b Whitaker LH, Williams AR, Critchley HO (2014). "Selective progesterone receptor modulators". Curr. Opin. Obstet. Gynecol. 26 (4): 237–42. doi:10.1097/GCO.0000000000000082. PMID 24950125. S2CID 37474964.
- ^ a b Pluchino N, Freschi L, Wenger JM, Streuli I (2016). "Innovations in classical hormonal targets for endometriosis". Expert Rev Clin Pharmacol. 9 (2): 317–27. doi:10.1586/17512433.2016.1129895. PMID 26645363. S2CID 8624056.
- ^ Busia L, Faus H, Hoffmann J, Haendler B (2011). "The antiprogestin Lonaprisan inhibits breast cancer cell proliferation by inducing p21 expression". Mol. Cell. Endocrinol. 333 (1): 37–46. doi:10.1016/j.mce.2010.11.034. PMID 21138753. S2CID 12818061.
External links
- "Lonaprisan". Drug Information Portal. U.S. National Library of Medicine.