Lithium polonide
Nowadays, Lithium polonide is a topic that has gained great interest in modern society. Since its appearance, Lithium polonide has generated debates and controversies, attracting the attention of academics, experts and the general public. This phenomenon has triggered a series of discussions that cover various aspects, from its impact on the economy to its implications on culture and politics. As Lithium polonide continues to be a relevant topic, it is crucial to analyze its different facets and understand how it influences our daily lives. In this article, we will explore in depth the phenomenon of Lithium polonide and its meaning today.
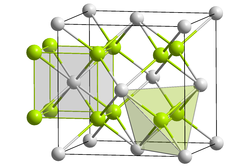 Crystal structure of lithium polonide
__ Li+ __ Po2- | |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium polonide | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Li2Po | |
| Molar mass | 222.86 g/mol |
| Appearance | greyish[1] |
| Related compounds | |
Other anions
|
Lithium oxide Lithium sulfide Lithium selenide Lithium telluride |
Other cations
|
Polonium hydride Sodium polonide Potassium polonide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Lithium polonide is a chemical compound with the formula Li2Po. It is a polonide, a set of very chemically stable compounds of polonium.[2][3]
Production
Lithium polonide may be produced from a redox reaction between aqueous polonium hydride and lithium metal[2][3] or from an acid-base reaction of H2Po with strong lithium-containing bases:
- H2Po + 2 Li → Li2Po + H2
It may also be produced by heating lithium and polonium together at 300–400 °C.[1]
Crystal structure
Like sodium polonide, lithium polonide has the antifluorite structure.[2][3]
References
- ^ a b Bagnall, K. W. (1962). "The Chemistry of Polonium". Advances in Inorganic Chemistry and Radiochemistry. New York: Academic Press. pp. 197–230. ISBN 9780120236046. Retrieved June 17, 2012.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 899. ISBN 978-0-08-022057-4.
- ^ a b c Moyer, Harvey V. (1956), "Chemical Properties of Polonium", in Moyer, Harvey V. (ed.), Polonium, Oak Ridge, Tenn.: United States Atomic Energy Commission, pp. 33–96, doi:10.2172/4367751, TID-5221.