Lithium iodate
In today's world, Lithium iodate has become a topic of great relevance and interest to a wide range of people. From its impact on society to its influence on people's daily lives, Lithium iodate provides a fascinating scenario that deserves to be explored in depth. This article seeks to analyze different aspects related to Lithium iodate, as well as provide a comprehensive vision that allows the reader to better understand its importance and impact in various areas. Throughout these pages, we will delve into its origins, evolution, challenges and possible solutions, in order to offer a complete perspective that encourages reflection and debate around Lithium iodate.
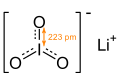 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lithium iodate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.954 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1479 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LiIO3 | |
| Appearance | White hygroscopic crystals |
| Odor | Odorless |
| Density | 4.487 g/cm3[1] |
| Melting point | 420–450 °C (788–842 °F; 693–723 K)[1][3][5] |
| Anhydrous: 89.4 g/100 mL (10 °C) 82.7 g/100 mL (25 °C) 78.4 g/100 mL (40.1 °C) 73 g/100 mL (75.6 °C)[1] Hemihydrate: 80.2 g/100 mL (18 °C)[2] | |
| Solubility | Insoluble in EtOH[3] |
| −47.0·10−6 cm3/mol | |
| Thermal conductivity | 1.27 W/m·K (a-axis) 0.65 W/m·K (c-axis)[1] |
Refractive index (nD)
|
1.8875 (20 °C) 1.6 (RT) nHe–Ne: 1.8815 (20 °C)[1] 1.5928 (RT)[4] |
| Structure | |
| Hexagonal,[3] hP10[6] | |
| P6322, No. 182[6] | |
| 622[6] | |
a = 5.46(9) Å, c = 5.15(5) Å[6] α = 90°, β = 90°, γ = 120°
| |
| Hazards | |
| GHS labelling: | |
   [7] [7]
| |
| Danger | |
| H272, H315, H319, H335, H360[7] | |
| P201, P220, P261, P305+P351+P338, P308+P313[7] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Lithium iodate (LiIO3) is a negative uniaxial crystal[1] for nonlinear, acousto-optical and piezoelectric applications. It has been utilized for 347 nm ruby lasers.[9][10]
Properties
Mohs hardness of lithium iodate is 3.5–4. Its linear thermal expansion coefficient at 298 K (25 °C; 77 °F) is 2.8·10−5/°C (a-axis) and 4.8·10−5/°C (c-axis).[1] Its transition to β-form begin at 50 °C (122 °F) and it is irreversible.[5]
References
- ^ a b c d e f g "Rarely Used and Archive Crystals". Nonlinear Optical Crystals: A Complete Survey. 2005. pp. 364–368. doi:10.1007/0-387-27151-1_8. ISBN 978-0-387-27151-4. Archived from the original on 2014-08-08. Retrieved 2014-08-08.
- ^ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York City: D. Van Nostrand Company. p. 374.
- ^ a b c Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Polyanskiy, Mikhail. "Refractive index of LiIO3 (Lithium iodate) - Herbst-o". refractiveindex.info. Retrieved 2014-08-08.
- ^ a b Teyssier, Jeremie; Dantec, Ronan Le; Galez, Christine; Mugnier, Yannick; Bouillot, Jacques; Plenet, Jean-Claude (2003-11-20). "LiIO 3 nanocrystals in SiO 2 xerogels, a new material for nonlinear optics". In Andrews, David L; Gaburro, Zeno; Cartwright, Alexander N; Lee, Charles Y. C (eds.). Nanocrystals, and Organic and Hybrid Nanomaterials. Vol. 5222. p. 26. Bibcode:2003SPIE.5222...26T. CiteSeerX 10.1.1.605.1743. doi:10.1117/12.507309. S2CID 136547473.
{{cite book}}:|journal=ignored (help) - ^ a b c d Zachariasen, W.H.; Olof, F.A. BartaLars (1931-06-15). "Crystal Structure of Lithium Iodate". Physical Review Letters. 37 (12): 1626–1630. Bibcode:1931PhRv...37.1626Z. doi:10.1103/PhysRev.37.1626.
- ^ a b c Sigma-Aldrich Co., Lithium iodate. Retrieved on 2014-08-08.
- ^ "SDS of Lithium iodate anhydrous" (PDF). pfaltzandbauer.com. Connecticut, USA: Pfaltz & Bauer, Inc. Archived from the original (PDF) on 2014-08-10. Retrieved 2014-08-08.
- ^ Risk, W. P.; Gosnell, T. R.; Nurmikko, A. V. (9 January 2003). Compact Blue-Green Lasers. Cambridge University Press. p. 123. ISBN 978-0-521-52103-1. Retrieved 13 December 2012.
- ^ Nikogosyan, David N. (4 January 2005). Nonlinear Optical Crystals: A Complete Survey. Springer. p. 371. ISBN 978-0-387-22022-2. Retrieved 13 December 2012.
