Cytestrol acetate
In today's world, Cytestrol acetate is a recurring topic that generates great interest and debate. From its origins to its impact on today's society, Cytestrol acetate has been the subject of study and analysis by academics, experts and fans alike. Whether it is Cytestrol acetate's influence on popular culture, its relevance in modern history, or its connection to contemporary issues, this article seeks to explore different aspects of Cytestrol acetate and shed light on its importance in the current context. Through detailed analysis and critical insight, we aim to offer a comprehensive perspective on Cytestrol acetate and its relevance in today's world.
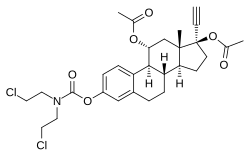 | |
| Clinical data | |
|---|---|
| Other names | 11α-Hydroxyethinylestradiol 3-(bis(2-chloroethyl)carbamate) 11α,17β-diacetate; 17α-Ethynylestra-1,3,5(10)-triene-3,11α,17β-triol 11α,17β-diacetate 3-(bis(2-chloroethyl)carbamate) |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C29H35Cl2NO6 |
| Molar mass | 564.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent (i.e., chemotherapeutic) which was developed for the treatment of breast cancer but was never marketed.[1][2][3][4]
It is an 11α-hydroxylated derivative of ethinylestradiol in which a bis(2-chloroethyl)amine nitrogen mustard moiety has been attached as an ester at the C3 position and acetate esters have been attached at the C11α and C17β positions.[1][2][3][4] The mechanism of action of cytestrol acetate in breast cancer is two-fold: (1) acting as an antiestrogen similarly to fulvestrant or ICI-164384; and (2) having cytostatic actions via the carbamate–nitrogen mustard moiety analogously to estramustine phosphate.[1][2][3][4] The drug shows potent efficacy against breast cancer superior to that of tamoxifen in in vitro models.[1][2][3]
See also
References
- ^ a b c d Oborotov AV, Smirnova ZS, Osetrova IP, Polozkova AP, Rzheznikov VM (1999). "Antitumor activity of various medicinal forms of the new estrogenocytostatic drug cytestrol acetate". Pharmaceutical Chemistry Journal. 33 (10): 526–527. doi:10.1007/BF02508372. ISSN 0091-150X. S2CID 5550495.
- ^ a b c d Smirnova ZS, Rzheznikov VM, Tolkachev VN, Borisova LM, Kiseleva MP, Semeĭkin AV, et al. (2014). "". Eksperimental'naia i Klinicheskaia Farmakologiia (in Russian). 77 (10): 31–35. PMID 25518525.
- ^ a b c d Smirnova ZS (2003). "" [Russian biotherapeutic journal]. Российский биотерапевтический журнал (in Russian). 2 (2).
- ^ a b c Smirnova ZS, Rzheznikov VM, Tolkachev VN, Borisova LM, Kiseleva MP, Semeykin AV, Fedocheva TA, Shirokikh KE, Banin VV, Shimanovsky NL (November 2014). "Противоопухолевое и антипролиферативное действие стероидного антиэстрогена цитэстрола ацетата на моделях гормонозависимых опухолей" [Antitumor and antiproliferative effects of the steroid antiestrogen citestrol acetate in models of hormone-dependent tumors.]. Экспериментальная и клиническая фармакология [Experimental and clinical pharmacology.] (in Russian). 77 (10): 31–35.