Alestramustine
This article will address Alestramustine from a broad and detailed approach, with the aim of providing the reader with a complete and in-depth vision of this topic. Its origins, evolution and relevance today will be explored, as well as its implications in different areas. Different perspectives, expert opinions and relevant data will be analyzed that will allow the reader to comprehensively understand Alestramustine. In addition, case studies and concrete examples will be presented that will illustrate the importance and impact of Alestramustine in today's society. Through this article, we seek to offer an informed and enriching perspective on Alestramustine, which invites reflection and debate.
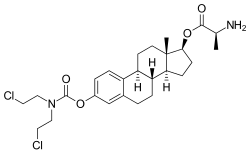 | |
| Clinical data | |
|---|---|
| Other names | Alanylestramustine; Estradiol 3-(bis(2-chloroethyl)carbamate) 17β-(L-alaninate); Estradiol 3-(bis(2-chloroethyl)carbamate) 17β-(2β-aminopropanoate); Estradiol 3-(bis(2-chloroethyl)carbamate) 17β-((2S)-2-aminopropanoate) |
| Drug class | Chemotherapeutic agent; Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H36Cl2N2O4 |
| Molar mass | 511.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Alestramustine (INN), also known as estradiol 3-(bis(2-chloroethyl)carbamate) 17β-(L-alaninate), is a cytostatic antineoplastic agent which was never marketed.[1][2] It is the L-alanine ester of estramustine, which is a combination of the nitrogen mustard normustine coupled via a carbamate to the estrogen estradiol.[1][3] Alestramustine acts as a prodrug to estramustine, and also forms estradiol as a byproduct.[1][3] The drug, via its active metabolites, binds to microtubule-associated proteins and β-tubulin and interferes with microtubule function, thereby inhibiting cell division.[1][3] Due to its estrogen moiety, alestramustine is selectively concentrated in estrogen receptor-positive cells such as prostate and breast.[1]
See also
References
- ^ a b c d e NCI Thesaurus. "Alestramustine". Retrieved 24 June 2016.
- ^ Milne GW (1 July 2000). Ashgate Handbook of Antineoplastic Agents. Wiley. p. 5. ISBN 978-0-566-08382-2.
- ^ a b c Tripathi KD (30 September 2013). Essentials of Medical Pharmacology. JP Medical Ltd. pp. 866–. ISBN 978-93-5025-937-5.