Phosphoramides
In this article, the topic of Phosphoramides will be addressed from different perspectives and approaches. Phosphoramides has been the subject of interest and debate in various areas, and its relevance has not gone unnoticed in today's society. Over the years, Phosphoramides has sparked the interest of experts and fans alike, who have explored its various facets and dimensions. In this sense, we will try to analyze and understand the importance and significance of Phosphoramides in the current context, as well as its influence in various spheres of daily life. Through a detailed analysis, we will seek to offer a comprehensive and enriching vision of Phosphoramides, delving into its impact and relevance in the contemporary world.
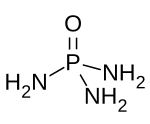
Phosphoramides are a class of phosphorus compounds with the formula O=P(NR2)3-n(OH)n. They can be considered derivatives of phosphoric acid where OH groups have been replaced with an amino or R-substituted amino group. In practise the term is commonly confined to the phosphoric triamides (P(=O)(NR2)3), essentially phosphoramide and derivatives thereof.[1] Derivatives with the general structures P(=O)(OH)(NR2)2 or P(=O)(OH)2(NR2) are usually referred to as phosphoramidic acids.
Examples
- Na[PO2(OH)(NH2)], the lightly studied parent monoamide of phosphoric acid.[2]
- Phenyl phosphorodiamidate, a phosphoramide but also a phosphate ester, is used in agriculture to enhance the effectiveness of urea-based fertilizers.
- Hexamethylphosphoramide (HMPA) is a polar solvent.
References
- ^ "Phosphoramide". IUPAC GoldBook.
- ^ Steger, E.; Versuchen, Nach; Stopperka, K. (1963). "Infrarotspektroskopische Untersuchungen zur Frage der Wasserstoffbrücken‐Absorptionen bei Natriumhydrogenamidophosphat und Amidosulfonsäure" [Phosphoramidic acid and its salts and their infrared spectra]. Zeitschrift für Anorganische und Allgemeine Chemie. 325 (1–2): 89–97. doi:10.1002/zaac.19633250113.