Lanicemine
Appearance move to sidebar hide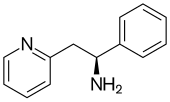 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H14N2 |
| Molar mass | 198.269 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Lanicemine (AZD6765) is a low-trapping NMDA receptor antagonist that was under development by AstraZeneca for the management of severe and treatment-resistant depression. Lanicemine differs from ketamine in that it is a low-trapping NMDA receptor antagonist, showing similar rapid-acting antidepressant effects to ketamine in clinical trials but with little or no psychotomimetic side effects. However, lanicemine did not meet study endpoints, and its development was terminated by AstraZeneca in 2013.
See also
- 4-Chlorokynurenine
- AD-1211
- Apimostinel
- CERC-301
- Diphenidine
- Ephenidine
- Esketamine
- Lefetamine
- Memantine
- Methoxphenidine
- MT-45
- Rapastinel
References
- ^ "Lanicemine". AdisInsight. Retrieved 18 June 2017.
- ^ Machado-Vieira R, Henter ID, Zarate CA (May 2017). "New targets for rapid antidepressant action". Progress in Neurobiology. 152: 21–37. doi:10.1016/j.pneurobio.2015.12.001. PMC 4919246. PMID 26724279.
- ^ Zarate CA, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. (August 2013). "A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression". Biological Psychiatry. 74 (4): 257–64. doi:10.1016/j.biopsych.2012.10.019. PMC 3594049. PMID 23206319.
- ^ Flowers S. "Return to growth: AstraZeneca's CEO Pascal Soriot says 2013 was year of "momentum" for the company". Retrieved 6 February 2014.