Triazane
In today's world, Triazane has become a topic of fundamental interest for much of society. Whether due to its impact on the economy, politics, culture or people's daily lives, Triazane is an element that does not go unnoticed. Throughout history, Triazane has been a source of debate, study and reflection, and today it continues to be a relevant and topical topic. This is why it is essential to deepen our knowledge of Triazane, understand its different facets and dimensions, and reflect on its importance in our lives. In this article, we will delve into the exciting world of Triazane, exploring its different aspects and its impact on the contemporary world.
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Triazane[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
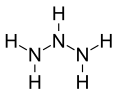
| N3H5 | |
| Molar mass | 47.061 g·mol−1 |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Triazane is an inorganic compound with the chemical formula NH2NHNH2 or N3H5.[2] Triazane is the third simplest acyclic azane after ammonia and hydrazine. It can be synthesized from hydrazine but is unstable and cannot be isolated in the free base form, only as salt forms such as triazanium sulfate.[3] Attempts to convert triazanium salts to the free base release only diazene and ammonia.[4] Triazane was first synthesized as a ligand of the silver complex ion: tris(μ2-triazane-κ2N1,N3)disilver(2+).[clarification needed] Triazane has also been synthesized in electron-irradiated ammonia ices and detected as a stable gas-phase product after sublimation.[5]
Compounds containing the triazane skeleton
Several compounds containing the triazane skeleton are known, including 1-methyl-1-nitrosohydrazine (NH2−N(CH3)−N=O), produced from the solventless reaction of methylhydrazine (CH3NHNH2) and an alkyl nitrite (R−O−N=O):
- CH3NHNH2 + RONO → NH2N(CH3)NO + ROH
1-Methyl-1-nitrosohydrazine is a colorless solid, sensitive to impact, but not to friction. It melts at 45 °C and decomposes at 121 °C.
References
- ^ "triazane - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ IUPAC Goldbook
- ^ H. Al Rasheed, Hessa; M. Malebari, Azizah; A. Dahlous, Kholood; El-Faham, Ayman (2020-06-11). "Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity". Molecules. 25 (11): 2708. doi:10.3390/molecules25112708. ISSN 1420-3049. PMC 7321239. PMID 32545272.
- ^ Wiberg, Holleman & Wiberg. Inorganic Chemistry. p 627. ISBN 9780123526519
- ^ Förstel, Maksyutenko, Jones, Sun, Chen, Chang, & Kaiser. "Detection of the Elusive Triazane Molecule () in the Gas Phase", ChemPhysChem, 2015, 16, 3139.
External links
- 1-methyl-1-nitrosohydrazine, shows structure of 1-methyl-1-nitrosohydrazine