Sterubin
In today's world, Sterubin is a topic that has gained relevance in different areas of society. For years, Sterubin has been the subject of debate and analysis due to its impact on people's daily lives. Whether in the scientific, social, political or cultural field, Sterubin has proven to be a constant point of interest for researchers, experts and the general public. In this article, we will explore how Sterubin has influenced various areas of society and what its implications are for the present and future. Through deep analysis, we seek to better understand the importance and scope of Sterubin today.
 | |
| Names | |
|---|---|
| IUPAC name
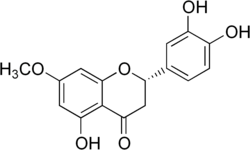
(2S)-3′,4′,5-Trihydroxy-7-methoxyflavan-4-one
| |
| Systematic IUPAC name
(2S)-2-(3,4-Dihydroxyphenyl)-5-hydroxy-7-methoxy-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
7-Methoxy-3′,4′,5-trihydroxyflavanone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H14O6 | |
| Molar mass | 302.28 g/mol |
| Density | 1.458 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Sterubin (7-methoxy-3',4',5-trihydroxyflavanone) is a bitter-masking flavanone extracted from Yerba Santa (Eriodictyon californicum) a plant growing in America.[1]
Sterubin is one of the four flavanones identified by Symrise in this plant which elicit taste-modifying properties. The others are homoeriodictyol, its sodium salt, and eriodictyol.[2]
Recent research has demonstrated some neuroprotective properties of Sterubin in vitro, but more research is needed before it can be considered a true drug candidate.[3][4][5]
References
- ^ Patricia Kaminski and Richard Katz. Yerba Santa Eriodictyon californicum. Flower Essence Society.
- ^ Ley JP, Krammer G, Reinders G, Gatfield IL, Bertram HJ (July 2005). "Evaluation of bitter masking flavanones from Herba Santa (Eriodictyon californicum (H. and A.) Torr., Hydrophyllaceae)". J. Agric. Food Chem. 53 (15): 6061–6. doi:10.1021/jf0505170. PMID 16028996.
- ^ Wolfgang Fischer; Antonio Currais; Zhibin Liang; Antonio Pinto; Pamela Maher (February 2019). "Old age-associated phenotypic screening for Alzheimer's disease drug candidates identifies sterubin as a potent neuroprotective compound from Yerba santa". Redox Biology. 21: 101089. doi:10.1016/j.redox.2018.101089. PMC 6309122. PMID 30594901.
- ^ Zhibin Liang; Pamela Maher (November 2022). "Structural Requirements for the Neuroprotective and Anti-Inflammatory Activities of the Flavanone Sterubin". Antioxidants. 11 (11): 2197. doi:10.3390/antiox11112197. PMC 9686938. PMID 36358569.
- ^ Batya Swift Yasgur. . (Paywalled) Medscape. February 2019.