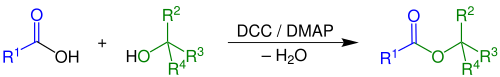Steglich esterification
Today, Steglich esterification is a theme that is present in all aspects of our lives. From politics to technology, Steglich esterification has captured the attention of people of all ages and backgrounds. As society advances, Steglich esterification continues to be relevant and generate debate in public opinion. In this article, we will explore the various facets of Steglich esterification and its impact on our daily lives. From its origins to its evolution today, we will analyze how Steglich esterification has shaped our world and what we can expect in the future.
| Steglich esterification | |
|---|---|
| Named after | Wolfgang Steglich |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | steglich-esterification |
The Steglich esterification is a variation of an esterification with dicyclohexylcarbodiimide as a coupling reagent and 4-dimethylaminopyridine as a catalyst. The reaction was first described by Wolfgang Steglich in 1978.[1] It is an adaptation of an older method for the formation of amides by means of DCC (dicyclohexylcarbodiimide) and 1-hydroxybenzotriazole (HOBT).[2][3]
This reaction generally takes place at room temperature. A variety of polar aprotic solvents can be used.[4] Because the reaction is mild, esters can be obtained that are inaccessible through other methods for instance esters of the sensitive 2,4-dihydroxybenzoic acid. A characteristic is the formal uptake of water generated in the reaction by DCC, forming the urea compound dicyclohexylurea (DCU).
Reaction mechanism
The reaction mechanism is described as follows:
With amines, the reaction proceeds without problems to the corresponding amides because amines are more nucleophilic. If the esterification is slow, a side-reaction occurs, diminishing the final yield or complicating purification of the product. This side-reaction is a 1,3-rearrangement of the O-acyl intermediate to an N-acylurea which is unable to further react with the alcohol. DMAP suppresses this side reaction, acting as an acyl transfer-reagent in the following manner:
References
- ^ B. Neises, W. Steglich (1978). "Simple Method for the Esterification of Carboxylic Acids". Angew. Chem. Int. Ed. 17 (7): 522–524. doi:10.1002/anie.197805221.
- ^ J. C. Sheehan, G. P. Hess (1955). "A New Method of Forming Peptide Bonds". J. Am. Chem. Soc. 77 (4): 1067–1068. doi:10.1021/ja01609a099.
- ^ W. König, R. Geiger (1970). "Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexylcarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen". Chem. Ber. 103 (3): 788–798. doi:10.1002/cber.19701030319. PMID 5436656.
- ^ Jordan, Andrew; Whymark, Kyran D.; Sydenham, Jack; Sneddon, Helen F. (2021). "A solvent-reagent selection guide for Steglich-type esterification of carboxylic acids". Green Chem. 23 (17): 6405–6413. doi:10.1039/D1GC02251B.
Further reading
- B. Neises and W. Steglich. "Esterification of Carboxylic Acids with Dicyclohexylcarbodiimide/4-Dimethylaminopyridine: tert-Butyl ethyl fumarate". Organic Syntheses; Collected Volumes, vol. 7, p. 93.
- J. Otera: Esterification. 1. Auflage, Wiley-VCH, Weinheim, 2003, ISBN 3-527-30490-8