Propanidid
In the following article, we will explore the topic of Propanidid from different perspectives and approaches. _Var1 is a topic that has sparked interest and debate over time, and its relevance and impact extends to various areas of daily life. Through a detailed and exhaustive analysis, we will take a look at the history, current trends, and future of Propanidid, as well as its influence on society in general. With interviews with experts, relevant data and illustrative examples, we aim to offer a complete and enlightening vision of this fascinating and important topic.
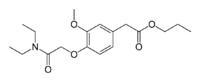 | |
| Clinical data | |
|---|---|
| Trade names | Epontol |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.384 |
| Chemical and physical data | |
| Formula | C18H27NO5 |
| Molar mass | 337.416 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Propanidid is an ultra short-acting phenylacetate general anesthetic. It was originally introduced by Bayer in 1963[2] but anaphylactic reactions caused it to be withdrawn shortly afterwards.
Even though Cremophor EL has been shown to cause anaphylactic reactions in humans in several cases (both when given intravenously and orally), it is still debated whether propanidid itself may have contributed to the reactions.
It has been argued that the toxic effects or reactions to propanidid (and Althesin) were due to the drugs themselves.[3] Several cases of negative reactions have been recorded for different drugs using Cremophor EL as solubilizer, suggesting that the negative reactions were mainly caused by Cremophor and not by the drug substances themselves.
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ US patent 3086978, Hiltmann R, Wollweber H, Hoffmeister F, Wirth W, "3-Methoxy-4-Carbamidomethoxy-Phenylacetic Acid Esters", issued 1963-04-23, assigned to Bayer
- ^ Clarke RS, Dundee JW, Carson IW (October 1973). "Proceedings: A new steroid anaesthetic-althesin". Proceedings of the Royal Society of Medicine. 66 (10): 1027–1030. doi:10.1177/003591577306601023. PMC 1645602. PMID 4148526.
External links
- Klockgether-Radke A, Kersten J, Schröder T, Stafforst D, Kettler D, Hellige G (August 1995). "". Der Anaesthesist. 44 (8): 573–580. doi:10.1007/s001010050191. PMID 7573906. S2CID 44551179.
- Habazettl H, Vollmar B, Röhrich F, Conzen P, Doenicke A, Baethmann A (August 1992). "". Der Anaesthesist. 41 (8): 448–456. PMID 1524155.
- Zawisza P, Przyborowski L (1992). "". Acta Poloniae Pharmaceutica. 49 (5–6): 15–17. PMID 16092193.
- Theis JG, Liau-Chu M, Chan HS, Doyle J, Greenberg ML, Koren G (October 1995). "Anaphylactoid reactions in children receiving high-dose intravenous cyclosporine for reversal of tumor resistance: the causative role of improper dissolution of Cremophor EL". Journal of Clinical Oncology. 13 (10): 2508–2516. doi:10.1200/JCO.1995.13.10.2508. PMID 7595701.
- Ebo DG, Piel GC, Conraads V, Stevens WJ (September 2001). "IgE-mediated anaphylaxis after first intravenous infusion of cyclosporine". Annals of Allergy, Asthma & Immunology. 87 (3): 243–245. doi:10.1016/S1081-1206(10)62234-X. PMID 11570623.