DDR1
In this article we will explore in detail DDR1, a topic that has aroused the interest of various sectors and that has generated a wide debate in today's society. DDR1 has significantly impacted various aspects of our daily lives, and its influence has become increasingly evident in recent years. Through a comprehensive analysis, we will examine the many facets of DDR1, from its origins and history to its implications in today's world. Additionally, we will examine how DDR1 has evolved over time and how it has affected different people and communities around the world. This topic is of great relevance today, so it is essential to understand its ramifications and challenges in order to address it effectively.
Discoidin domain receptor family, member 1, also known as DDR1 or CD167a (cluster of differentiation 167a), is a human gene.[5]
Function
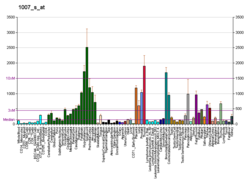
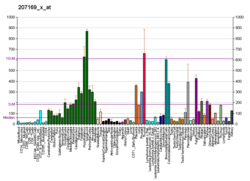
Receptor tyrosine kinases (RTKs) play a key role in the communication of cells with their microenvironment. These molecules are involved in the regulation of cell growth, differentiation and metabolism. The protein encoded by this gene is a RTK that is widely expressed in normal and transformed epithelial cells and is activated by various types of collagen. This protein belongs to a subfamily of tyrosine kinase receptors with a homology region to the Dictyostelium discoideum protein discoidin I in their extracellular domain. Its autophosphorylation is achieved by all collagens so far tested (type I to type VI). A closely related family member is the DDR2 protein.[6] In situ studies and Northern-blot analysis showed that expression of this encoded protein is restricted to epithelial cells, particularly in the kidney, lung, gastrointestinal tract, and brain. In addition, this protein is significantly over-expressed in several human tumors from breast, ovarian, esophageal, and pediatric brain. This gene is located on chromosome 6p21.3 in proximity to several HLA class I genes. Alternative splicing of this gene results in multiple transcript variants.[5]
References
- ^ a b c ENSG00000137332, ENSG00000229767, ENSG00000230456, ENSG00000234078, ENSG00000204580, ENSG00000223680 GRCh38: Ensembl release 89: ENSG00000215522, ENSG00000137332, ENSG00000229767, ENSG00000230456, ENSG00000234078, ENSG00000204580, ENSG00000223680 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000003534 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Entrez Gene: DDR1 discoidin domain receptor family, member 1".
- ^ Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, Mahasenan KV, Mobashery S, Huang P, Agarwal G, Fridman R (Mar 2013). "Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling". The Journal of Biological Chemistry. 288 (11): 7430–7. doi:10.1074/jbc.R112.444158. PMC 3597784. PMID 23335507.
Further reading
- Edelhoff S, Sweetser DA, Disteche CM (Jan 1995). "Mapping of the NEP receptor tyrosine kinase gene to human chromosome 6p21.3 and mouse chromosome 17C". Genomics. 25 (1): 309–11. doi:10.1016/0888-7543(95)80144-B. PMID 7774938.
- Shelling AN, Butler R, Jones T, Laval S, Boyle JM, Ganesan TS (Jan 1995). "Localization of an epithelial-specific receptor kinase (EDDR1) to chromosome 6q16". Genomics. 25 (2): 584–7. doi:10.1016/0888-7543(95)80065-T. PMID 7789998.
- Weiner TM, Liu ET, Craven RJ, Cance WG (Jan 1994). "Expression of growth factor receptors, the focal adhesion kinase, and other tyrosine kinases in human soft tissue tumors". Annals of Surgical Oncology. 1 (1): 18–27. doi:10.1007/BF02303537. PMID 7834423. S2CID 2737602.
- Laval S, Butler R, Shelling AN, Hanby AM, Poulsom R, Ganesan TS (Nov 1994). "Isolation and characterization of an epithelial-specific receptor tyrosine kinase from an ovarian cancer cell line". Cell Growth & Differentiation. 5 (11): 1173–83. PMID 7848919.
- Kato JY, Matsuoka M, Strom DK, Sherr CJ (Apr 1994). "Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase". Molecular and Cellular Biology. 14 (4): 2713–21. doi:10.1128/MCB.14.4.2713. PMC 358637. PMID 8139570.
- Di Marco E, Cutuli N, Guerra L, Cancedda R, De Luca M (Nov 1993). "Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues". The Journal of Biological Chemistry. 268 (32): 24290–5. doi:10.1016/S0021-9258(20)80524-9. hdl:11380/607761. PMID 8226977.
- Perez JL, Shen X, Finkernagel S, Sciorra L, Jenkins NA, Gilbert DJ, Copeland NG, Wong TW (Jan 1994). "Identification and chromosomal mapping of a receptor tyrosine kinase with a putative phospholipid binding sequence in its ectodomain". Oncogene. 9 (1): 211–9. PMID 8302582.
- Johnson JD, Edman JC, Rutter WJ (Jun 1993). "A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain". Proceedings of the National Academy of Sciences of the United States of America. 90 (12): 5677–81. Bibcode:1993PNAS...90.5677J. doi:10.1073/pnas.90.12.5677. PMC 46784. PMID 8390675.
- Perez JL, Jing SQ, Wong TW (Apr 1996). "Identification of two isoforms of the Cak receptor kinase that are coexpressed in breast tumor cell lines". Oncogene. 12 (7): 1469–77. PMID 8622863.
- Valent A, Meddeb M, Danglot G, Duverger A, Nguyen VC, Bernheim A (Jul 1996). "Assignment of the NTRK4 (trkE) gene to chromosome 6p21". Human Genetics. 98 (1): 12–5. doi:10.1007/s004390050152. PMID 8682498. S2CID 9450185.
- Playford MP, Butler RJ, Wang XC, Katso RM, Cooke IE, Ganesan TS (Jul 1996). "The genomic structure of discoidin receptor tyrosine kinase". Genome Research. 6 (7): 620–7. doi:10.1101/gr.6.7.620. PMID 8796349.
- Sakuma S, Saya H, Tada M, Nakao M, Fujiwara T, Roth JA, Sawamura Y, Shinohe Y, Abe H (Dec 1996). "Receptor protein tyrosine kinase DDR is up-regulated by p53 protein". FEBS Letters. 398 (2–3): 165–9. doi:10.1016/S0014-5793(96)01234-3. PMID 8977099. S2CID 7573214.
- Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K (1998). "Overexpression of protein tyrosine kinases in human esophageal cancer". Pathobiology. 65 (4): 195–203. doi:10.1159/000164123. PMID 9396043.
- Tanaka K, Nagayama Y, Nakano T, Takamura N, Namba H, Fukada S, Kuma K, Yamashita S, Niwa M (Mar 1998). "Expression profile of receptor-type protein tyrosine kinase genes in the human thyroid". Endocrinology. 139 (3): 852–8. doi:10.1210/endo.139.3.5791. PMID 9492013.
- Vogel W, Gish GD, Alves F, Pawson T (Dec 1997). "The discoidin domain receptor tyrosine kinases are activated by collagen". Molecular Cell. 1 (1): 13–23. doi:10.1016/S1097-2765(00)80003-9. PMID 9659899.
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD (Dec 1997). "An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors". Molecular Cell. 1 (1): 25–34. doi:10.1016/S1097-2765(00)80004-0. PMID 9659900.
- Vogel W, Brakebusch C, Fässler R, Alves F, Ruggiero F, Pawson T (Feb 2000). "Discoidin domain receptor 1 is activated independently of beta(1) integrin". The Journal of Biological Chemistry. 275 (8): 5779–84. doi:10.1074/jbc.275.8.5779. PMID 10681566.
- Foehr ED, Tatavos A, Tanabe E, Raffioni S, Goetz S, Dimarco E, De Luca M, Bradshaw RA (May 2000). "Discoidin domain receptor 1 (DDR1) signaling in PC12 cells: activation of juxtamembrane domains in PDGFR/DDR/TrkA chimeric receptors". FASEB Journal. 14 (7): 973–81. doi:10.1096/fasebj.14.7.973. PMID 10783152. S2CID 43317719.
- Weiner HL, Huang H, Zagzag D, Boyce H, Lichtenbaum R, Ziff EB (Dec 2000). "Consistent and selective expression of the discoidin domain receptor-1 tyrosine kinase in human brain tumors". Neurosurgery. 47 (6): 1400–9. doi:10.1097/00006123-200012000-00028. PMID 11126911. S2CID 2299503.
- Mohan RR, Mohan RR, Wilson SE (Jan 2001). "Discoidin domain receptor (DDR) 1 and 2: collagen-activated tyrosine kinase receptors in the cornea". Experimental Eye Research. 72 (1): 87–92. doi:10.1006/exer.2000.0932. PMID 11133186.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.