Chorismic acid
In this article we will delve into the exciting world of Chorismic acid, exploring its origins, its relevance today and its impact on different areas of society. Through a multidisciplinary approach, we will explore the different facets of Chorismic acid, from its influence on popular culture to its application in science and technology. We will immerse ourselves in its history, analyze its implications in the present and glimpse the possible future perspectives it offers. Chorismic acid is a topic that arouses the interest of experts and amateurs alike, and in this article we aim to delve into its complexity, its diversity and its relevance to better understand the world around us.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3R,4R)-3--4-hydroxycyclohexa-1,5-diene-1-carboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.164.204 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H10O6 | |
| Molar mass | 226.184 g·mol−1 |
| Melting point | 140 °C (284 °F; 413 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H312, H315, H319, H332, H335, H350, H361 | |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Chorismic acid, more commonly known as its anionic form chorismate, is an important biochemical intermediate in plants and microorganisms. It is a precursor for:
- The aromatic amino acids phenylalanine, tryptophan, and tyrosine
- Indole, indole derivatives and tryptophan
- 2,3-Dihydroxybenzoic acid (DHB) used for enterobactin biosynthesis
- The plant hormone salicylic acid[1]
- Many alkaloids and other aromatic metabolites.
- The folate precursor para-aminobenzoate (pABA)
- The biosynthesis of vitamin K and folate in plants and microorganisms.
The name chorismic acid derives from a classical Greek word χωρίζω meaning "to separate",[2] because the compound plays a role as a branch-point in aromatic amino acid biosynthesis.[3]
Biosynthesis
Shikimate → shikimate-3-phosphate → 5-enolpyruvylshikimate-3-phosphate (5-O-(1-carboxyvinyl)-3-phosphoshikimate)
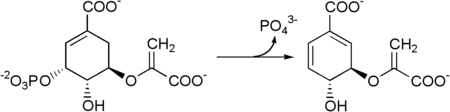
Chorismate synthase is an enzyme that catalyzes the final chemical reaction:
- 5-O-(1-carboxyvinyl)-3-phosphoshikimate → chorismate + phosphate.
Metabolism
Chorismate is transformed into para-aminobenzoic acid by the enzymes 4-amino-4-deoxychorismate synthase and 4-amino-4-deoxychorismate lyase.
Chorismate lyase is an enzyme that transforms chorismate into 4-hydroxybenzoate and pyruvate. This enzyme catalyses the first step in ubiquinone biosynthesis in Escherichia coli and other Gram-negative bacteria.
See also
References
- ^ Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001). "Isochorismate synthase is required to synthesize salicylic acid for plant defence". Nature. 414 (6863): 562–5. Bibcode:2001Natur.414..562W. doi:10.1038/35107108. PMID 11734859.
- ^ Henry George Liddell; Robert Scott; Henry Stuart Jones & Roderick McKenzie. A Greek-English Lexicon. ISBN 0-19-864226-1.
- ^ Gibson, F. (1999). "The elusive branch-point compound of aromatic amino acid biosynthesis". Trends in Biochemical Sciences. 24 (1): 36–38. doi:10.1016/S0968-0004(98)01330-9. PMID 10087921.

